Beef Is When I See You
Smoked meats often have a pink layer directly below the surface, nestled neatly under the bark. Called the smoke ring, it has long been an emblem of great barbecue. Backyarders know they have arrived when they make their first smoke ring. BBQ judges eat with their eyes and look for smoke rings at competitions.
Alas, as with so many things barbecue, there is a lot of misinformation about the smoke ring out there, and anybody who tells you that good barbecue needs a smoke ring is blowing smoke. The AmazingRibs.com science advisor Prof. Greg Blonder,has done extensive research for us on the subject and has pinned down the facts, among them, you don't even need smoke to make a smoke ring!
It's all about the myoglobin
Smoke can be described, a bit simplistically, as tiny particles that we can see mixed with water vapor, and gases (click here to read more about smoke). Blonder has proven that the smoke ring is an interaction between a pink protein in meat named myoglobin with the gases nitric oxide (NO) and carbon monoxide (CO). NO and CO are made by the combination of carbon and nitrogen with oxygen during the combustion of wood or charcoal. The white billowy stuff has nothing to do with the process.
Here's how it works. Muscles use oxygen and other compounds as a fuel. A protein called hemoglobin carries oxygen from the lungs through the blood stream to the walls of the muscle fibers where it passes into the cells and is glommed onto by myoglobin. Myoglobin is suspended in water and stores the oxygen until it is needed. When an animal is slaughtered, the blood and hemoglobin are drained, but much of the myoglobin water, called myowater, remains trapped in the fibers. It accounts for about 75% of the weight of meat and much of the moisture we feel when we eat. When you buy meat, the pink liquid in the package is not blood, it is myowater.
Myoglobin is rusty colored because it has an iron compound called a heme and it is responsible for most of the color of meat. Some muscles have more myoglobin than others and that makes them pinker. Beef has more than turkey dark meat which has more than turkey white meat. But all three give up pink juice. Dr. Blonder explains that "In high oxygen environments, like air on the surface of freshly cut meat, myoglobin is bright red. In zero oxygen environments, like deep inside a steak, myoglobin is purplish. In low oxygen environments, as in week-old packaged meats, myoglobin is brown. As you might imagine, this color change is reversible because myoglobin is designed to continually shuttle oxygen back and forth through the cell wall. So a raw gray meat surface is reversible to pink on exposure to air." Cut open a package of gray steak and it will often pink up with exposure to oxygen. Cut open a steak on the grill to see if it is done, and when you bring it to the table, it will be a different color as oxygen combines with the myoglobin (yet another argument for digital thermometers).
NO and CO are not very stable so they willingly mix it up with wet meat juices and basting liquids on the surface. These dissolved gases react with the iron in the myoglobin and prevent it from changing color when the meat is cold. Some meat packers are now injecting CO into the packaging to keep the meat bright pink longer.
Like many proteins, myoglobin changes color permanently when it breaks down after exposure to heat. In beef the meat goes from purple to red to pink to gray at very specific temperatures, and that is what defines rare, medium rare, well done, etc. Once myoglobin breaks down, at about 170°F in beef and 100°F in tuna, the game is over, it cannot return to pink.
While meat is starting to cook, if NO or CO land on the surface and dissolve into the meat, they "fix" the color pink while the rest of the meat heats up and goes to gray. But NO and CO cannot diffuse very far beyond the surface before the meat beneath it heats up, dooming the myoglobin in the interior to a colorless fate. As a result, the pink forms a thin layer, the smoke ring, which usually only goes about 1/8″ deep, although, under some circumstances, it can go up to 1/2″ deep.
This chemical reaction is similar to the one that occurs when you cure meat by sprinkling it with a curing salt containing sodium nitrite. These are the salts that give bacon, hot dogs, and corned beef their characteristic pink color. They lock in the pink color. Sprinkle a little on meat and bake it in an indoor oven and voila, smoke ring. So you don't need smoke to make a smoke ring, you just need NO or CO. To prove the point Dr. Blonder placed pork tenderloins in five kinds of filter bags: Parchment paper, a kraft paper bag, butcher paper, a Reynold's turkey basting bag, and a HEPA (high-efficiency particulate air) filter you might buy for a shopvac. They are porous enough to let gases through, but not the much larger smoke particles.

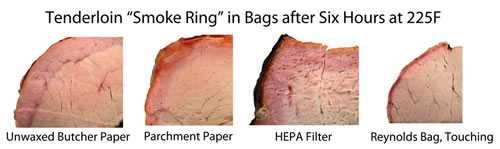
As you can see, all four meats had distinct smoke rings. So no puffy white clouds are necessary. The process is very complex and depends on a number of variables including humidity, oxygen levels, combustion temp, how dry the wood is, and even the pH of the meat itself. But NO and CO are crucial ingredients and how much you get depends a lot on your smoker. Here's a table Dr. Blonder created from actual readings he took with sensitive equipment (and no he did not start a savannah fire, he simulated it in a fire ring in his yard).
| Fuel | NO in exhaust |
|---|---|
| Savannah grass fire, near surface | 250 ppm |
| Roaring Kingsford charcoal briquets, no wood | 100-200 ppm |
| Roaring wood fire (depends on wood) | 50-200 ppm |
| Pellet smoker at 225°F | 25-50 ppm |
| Roaring lump charcoal (depends on wood and brand) | 10-70 ppm |
| Charcoal briquets, long slow cook at about 225°F | <20 ppm |
| Propane smoker, hot flame, no wood | <20 ppm |
| Smokenator, no wood (indirect Weber Kettle with briquets) | <10 ppm |
| Electric smoker with wood lumps | <2 ppm |
| Propane grill, no wood | <2 ppm |
This data is quite startling. Plain charcoal briquets, roaring hot, produce about the same amount of NO as plain wood and significantly more than lump charcoal. Lump charcoal is almost pure carbon, and thus deficient in nitrogen compared to briquets which contain partially carbonized sawdust and actual coal dust, along with other additives. In addition, lump produces less NO because the irregular shapes interlock and block airflow, while briquets, with their grooved and rounded tops and bottoms allow more oxygen in so they actually burn hotter, contrary to popular opinion, and produces more NO.
A propane grill burns extremely efficiently producing little more than carbon dioxide and water as byproducts. Electric smokers are low power, produce little energy compared to full out combustion, and there is little airflow since oxygen is not sucked in as a fuel, so the wood smolders rather than burning, producing little NO. Instead of burning at 1,600°F as in a top of the line offset smoker, wood hunks in an electric smolder between 400°F and 900°F, according to Blonder.
To prove it, in a complicated and dangerous series of experiments, Dr. Blonder cooked meat in a container filled with an NO solution, and another in CO. Both developed smoke rings.
The thickness of the smoke ring also depends on the fat cap. If you leave on the layer of fat, NO and CO will penetrate into it, but the fat doesn't contain myoglobin, so it doesn't have any pink to be fixed. So the smoke ring will form under the fat if the gases penetrate that far. But if the fat layer is thick, the NO and CO will not get through and there will be no smoke ring. Yet another reason to remove most of the fat.
The locking of the myoglobin color starts almost immediately if you have a good stable clean fire. If you have a charcoal wood, or pellet fire, try this: Put a slab of ribs on at 225°F. After 30 minutes, move it indoors and finish cooking. There will be a fine looking smoke ring. After just 30 minutes exposed to NO and CO!
It has long been known that smoke rings stop growing as the cook progresses. The point at which they stop seems to be when the myoglobin hits about 170°F depending on a number of variables. At that temp it has broken down so far that the pink color can no longer be locked in.
How to get a smoke ring
All this research busts a bunch of myths. The smoke ring is not caused by the billowy white stuff, it is caused by gases in the smoke. It is not enhanced by paprika. There is no time limit on smoke absorption. The ring stops growing when the meat hits about 170°F and myoglobin loses its oxygen retaining ability, not 140°F. Salt has little to do with it.
The amount of myoglobin in the meat is important. Beef has more than pork which has more than chicken. Older animals have more than youngsters. Fresh kills have more. Hard working muscles have more.
Moisture. Keep the surface wet by basting or spritzing it with a thin water based mop. In many parts of the country mopping with vinegar based liquids is popular. Many people spritz with apple juice, which also has fructose which can help with browning. Blonder explains: "First, when water evaporates from the surface of the meat it cools the meat and this enhances condensation of NO. Second, the water is 'sticky' and grabs onto passing smoke chemicals. And third, it delays the formation of a dense bark which impedes absorption of smoke chemicals."
When smoke roasting, moist meat holds onto smoke more readily than dry meat. Less smoke sticks as the cooking continues because the surface of the meat begins to dry. For this reason putting a pan of water in a smoker helps create a smoke ring because the evaporating water condenses on the meat. In fact some smokers, called water smokers, have water pans built in. The Weber Smokey Mountain is the best known of this breed.
As smoke particles and combustion gases land on the surface of meat, especially cool moist meat from the fridge, they condense, dissolve, and some are moved deeper into the meat by diffusion and absorption. The cells are simply seeking equilibrium. The process is the same as when someone lights a cigar in a room. All the smoke starts out near the cigar, but eventually it spreads throughout the room as it achieves equilibrium. After a while it penetrates clothes, furniture, and even food. Because it is water soluble, cigar smoke will get into wet things first, like your wife's eyes. Before long you and your cigar will be seeking equilibrium in the garage.
Temperature of the meat. Start with cold meat and keep the temp down at first. Then basting or spritzing the surface cools it and allows the ring to grow. Yet another reason to cook low and slow. When meat is cooked hot, the myoglobin turns brown faster, so the NO and CO don't penetrate deeply before the meat changes color. When cooked slowly, the muscle proteins finish breaking down before the naturally pink myoglobin denatures and so the meat remains pink. You can occasionally see this phenomenon in braised meat like a beef stew. It may have been cooked for hours in a liquid at low temps, yet the meat will still be slightly pink inside.
Burn wood.Burning wood and humid air produce more of a smoke ring.
Don't use electric smokers. That is partially because the wood smolders at a low temp in electrics, and high temps are required to create the NO and CO. Experts at cooking in electric smokers sometimes add a charcoal briquet as well as wood to create the correct atmospheric conditions for a smoke ring. Some of these briquets actually contain powdered sodium nitrates, which enhance ring formation. But in general, a vigorous charcoal or wood fire at just the right temperature, produces the deepest ring and the best meat. Click here to learn more about wood and smoke.
Remove the fat. NO and CO can penetrate fat, but if the underlying meat changes color before they get to it, you won't have a smoke ring. There are many of other good reasons to get rid of the fat cap.
Dr. Blonder, the scientist, in a poetic moment observes that "Myoglobin, a molecule essential for life, is as fragile as life itself. It can breathe in gases, and react by changing color. It can die when it overheats. And like every individual, it has its own, unique personality. Understanding myoglobin is a key to understanding how meat cooks, looks and tastes." Click here to read more of the science and Dr. Blonder's tests.
Source: https://amazingribs.com/more-technique-and-science/more-cooking-science/mythbusting-smoke-ring-no-smoke-necessary/
0 Response to "Beef Is When I See You"
Post a Comment